
An air clean room, often simply called a cleanroom, is a controlled environment engineered to maintain extremely low levels of airborne particles such as dust, microbes, aerosol particles, and chemical vapors. These rooms are critical in industries where even microscopic contamination can affect product integrity—such as semiconductor manufacturing, pharmaceuticals, aerospace, and biotechnology.
What Does "Air Clean Room" Actually Mean?
A cleanroom is defined by ISO 14644-1 as a room in which the concentration of airborne particles is controlled and classified, and where additional parameters such as temperature, humidity, air pressure, and airflow patterns are also precisely regulated.
Unlike regular rooms, air cleanrooms use advanced HEPA (High-Efficiency Particulate Air) or ULPA (Ultra-Low Penetration Air) filters to remove up to 99.99% of airborne particles, with air constantly recirculated to maintain ultra-clean conditions.
Why Are Cleanrooms Important?
Air cleanrooms play a vital role in:
Ensuring product quality in microelectronics and pharmaceutical production.
Preventing contamination in food and medical device manufacturing.
Protecting sensitive research in life sciences and space exploration.
Even a single speck of dust can ruin a semiconductor wafer or contaminate a sterile drug vial, which is why air quality control is non-negotiable in cleanroom operations.

ISO Cleanroom Classifications (ISO 1 to ISO 9)
Cleanrooms are categorized by how clean the air is, based on the number and size of particles per cubic meter.
| ISO Class | ≥0.5 μm particles/m³ | Typical Application |
|---|---|---|
| ISO 1 | 10 | Nanotech research |
| ISO 5 | 3,520 | Semiconductor manufacturing |
| ISO 7 | 352,000 | Biotech, pharmaceutical filling rooms |
| ISO 9 | 35,200,000 | Normal indoor room air |
The lower the ISO number, the cleaner the room. Source: ISO 14644-1
How Airflow and Filtration Work in Cleanrooms
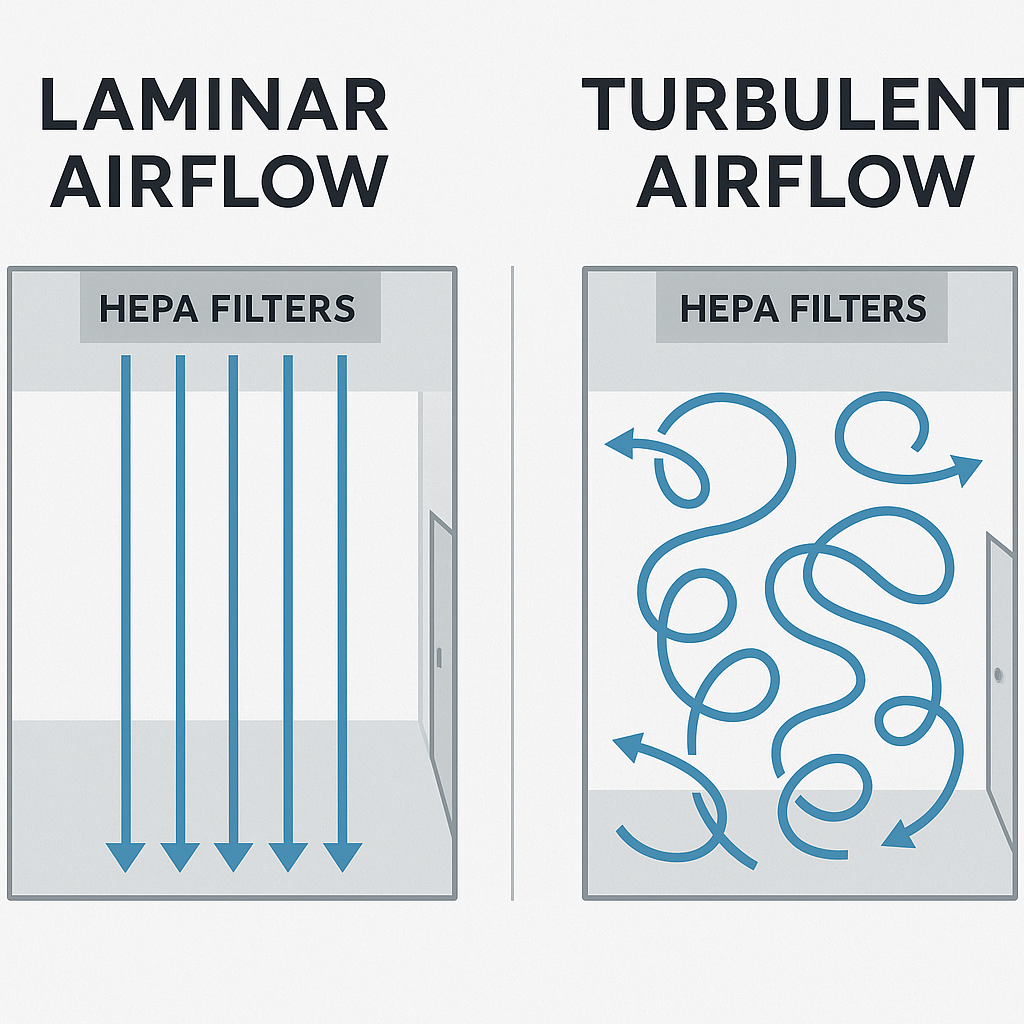
To achieve and maintain cleanliness levels, air cleanrooms use three core systems:
HEPA or ULPA Filtration Units
These capture fine particles (≥0.3 µm or even smaller) and are typically installed in ceilings or fan-filter units (FFUs).Airflow Design
Laminar Flow (Unidirectional): Smooth, vertical or horizontal airflow that sweeps particles away.
Turbulent Flow (Non-unidirectional): Mixed air circulation used in lower-grade cleanrooms.
Air Changes Per Hour (ACH)
Cleanrooms replace air up to 600 times per hour, depending on the required class. This rapid exchange dilutes and removes contaminants.
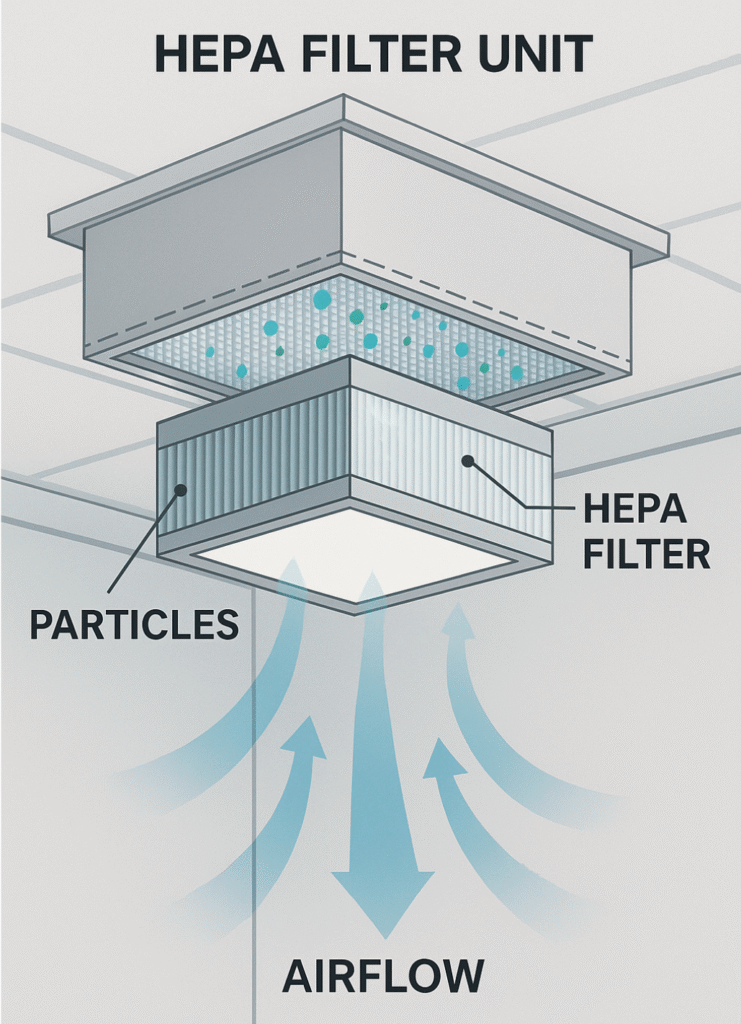
In ISO Class 5 rooms, airspeed is typically maintained between 0.3 to 0.5 m/s, moving vertically downward from ceiling to floor.
Design Elements of an Air Clean Room
To preserve its sterile environment, a cleanroom must be carefully constructed using:
Low-shedding materials like stainless steel or coated aluminum
Air-tight panel systems, epoxy-sealed floors, and interlocked cleanroom doors
Positive or negative pressure systems depending on the application:
Positive pressure: Keeps outside contaminants out (e.g., electronics, pharma)
Negative pressure: Contains contaminants within (e.g., BSL labs)
Additional features may include:
Air showers for personnel entry
Sticky mats, cleanroom garments, and GMP-compliant cleaning protocols
Real-World Applications
Air cleanrooms are used in a wide range of industries:
Semiconductors & Microelectronics
Pharmaceutical Manufacturing
Biotechnology & Medical Devices
Aerospace and Optics
Food and Beverage Packaging
Solar Panel Production
Each application demands a different ISO classification depending on sensitivity.
Final Thoughts
An air cleanroom isn’t just about filtered air—it’s a precise, highly engineered ecosystem that upholds the highest standards of cleanliness, safety, and compliance. From the ceiling-mounted filters to the personnel protocols, every detail is tuned to protect products, processes, and people.
Frequently Asked Questions (FAQ)
1. What is the purpose of a cleanroom?
To minimize the introduction, generation, and retention of airborne particles that may compromise sensitive processes or products.
2. How is a cleanroom air classified?
By ISO 14644-1, which defines allowable particle counts by size per cubic meter of air.
3. What's the difference between an operating room and a cleanroom?
Operating rooms focus on biological safety, while cleanrooms control particle and chemical contamination. Some hybrid rooms meet both standards.
4. What qualifies as a cleanroom?
A sealed environment equipped with air filtration (HEPA/ULPA), regulated pressure, temperature, and humidity, designed to meet specific ISO class limits.

